Stepwise Process for Improving the Quality of HIV Rapid and Recency Testing (SPI-RRT)
To assist ministries of health and national programs, a Stepwise Process for Improving the Quality of HIV Rapid and Recency Testing (SPI-RRT) checklist has been developed. The checklist provides guidance on quality assurance (QA) practices for sites using HIV rapid tests to diagnose HIV infection and for sites using the rapid test for recent infection (RTRI) for HIV-1 recent infection surveillance. The SPI-RRT checklist sets minimum standards for all HIV RT/RTRI testing points and provides guidelines for continuous quality improvement (CQI).
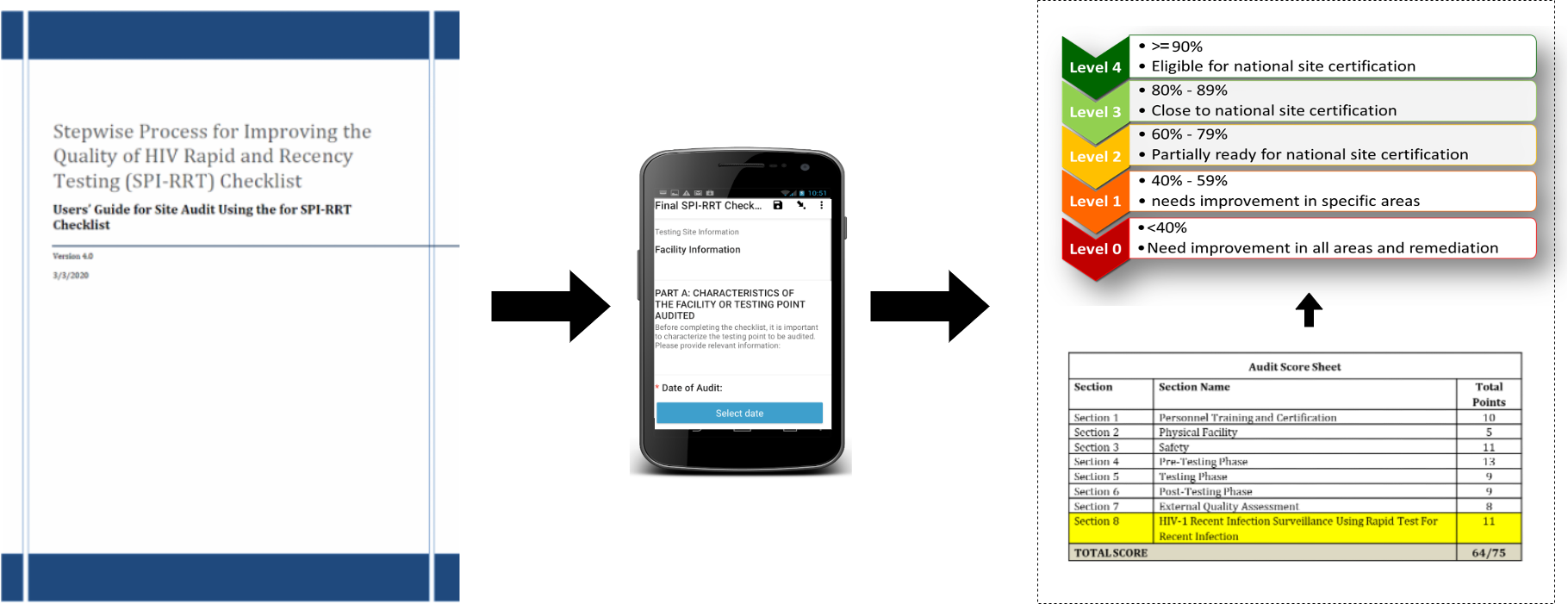

Working through the SPI-RRT Checklist will enable the individuals in charge of the HIV RRT/RTRI testing points and facilities to recognize quality gaps and shortcomings, identify areas for improvement and where additional resources may be needed to achieve national certification. Using the SPI-RRT checklist, the HIV rapid and recency testing site audits are intended to be effective means to:
- determine if a testing facility is providing accurate and reliable results.
- determine if HIV RT/RTRI testing facilities are well-managed and is adhering to quality practices.
- identify areas for improvement.