Rapid Test Continuous Quality Improvement
The current initiative is to expand HIV testing to achieve UNAIDS 95-95-95 targets. There has been significant progress meeting these targets and many countries are reaching epidemic control. It is both a programmatic and ethical imperative and priority for Ministries of Health (MOH) and National AIDS Control (NAC) programs to implement robust quality management systems that deliver reliable and accurate HIV test results. The HIV Rapid Test Continuous Quality Improvement initiative (RTCQI), launched in 2014 as a practical and comprehensive approach to ensure the quality of HIV test results.
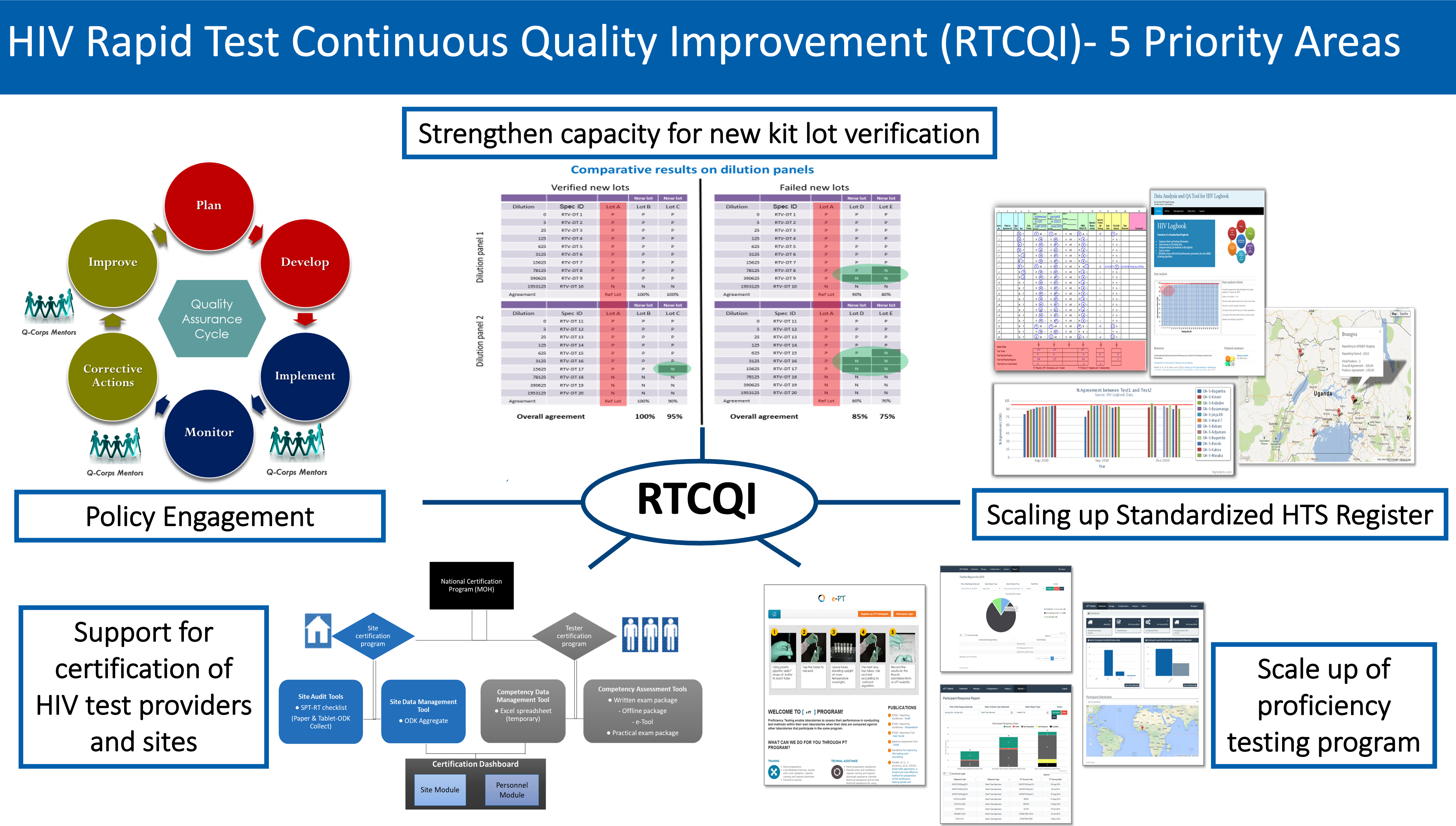
RTCQI is based on five main pillars which include
- Supporting countries to develop policies and implement guidelines on quality assurance for HIV related point of care testing
- Strengthening human resources through training and competency based qualification of testers
- Implementing a stepwise process for improving the quality of testing towards sites certification
- Completing the quality assurance cycle for standardized HTS register and proficiency testing (PT) program in order to increase uptake
- Strengthening national capacity for rapid test kit lot verification or post market surveillance (PMS)
RTCQI emphasizes the quality assurance cycle (QAC) which is a foundation for increasing uptake, coverage and impact of quality assurance for HIV testing. The QAC is comprised of three major phases including: Planning, Implementation, and Evaluation. Conceptualized in a cycle, the emphasis on the QAC is to ensure all activities of the various phases are completed and RTCQI is guided by the implementation of innovative strategies to ensure the QAC is accomplished.
RTCQI Implementation Subject Matter Experts (SMEs)
The people who make RTCQI implementations happen across the world.
Mireille B. Kalou

Mireille B. Kalou is a medical doctor with a master’s degree in public health – Infectious Disease. Mireille has over 20 years of experience in laboratory science, capacity building, program design and management. Mireille had spearheaded and laid cornerstones to the PEPFAR centrally funded HIV Rapid Test Quality Improvement Initiative while supporting its adoption by ministries of health in multiple countries in African, Asia and the Caribbean Region. Since 2018 she has been serving as senior Laboratory Advisor to oversee the PEPFAR lab portfolio of Haiti (2018-2021) and Eswatini (2022 to present). In that capacity, she has collaborated with laboratory stakeholders in countries to strengthen laboratory services and systems, develop national strategic plans, provide technical guidance for the establishment of the national public health laboratory. In addition, she is supporting the MoH Port Health team in their efforts to strengthen border health capacity for screening travelers in and out of Eswatini. As the new activity manager for the Ministry of Health Cooperative Agreement, she chaired an PEPFAR interagency task team to align two government-to-government funding mechanisms. She has been recognized several times for her outstanding contributions. She has received the American Embassy Mission Honor Award in Cameroon, the Mission National Meritorious Honor Award in Haiti and in Eswatini, and the Center for Global Health Excellence in Laboratory Quality Award. She has authored and co-authored more than 20 articles in peer-reviewed journals and book chapter. In her free time, Mireille spends quality time with her daughter, enjoys baking, walking, relaxing with a nice book and whenever possible, discovering new places.
Trudy Dobbs

Trudy Dobbs is currently a Biologist at the CDC’s Division of Global HIV and TB (DGHT).
Trudy has over 35 years of experience in strengthening HIV diagnostic quality, from her early career in the Newborn Screening Quality Assurance (QA) Program supporting QA measures for HIV surveillance in childbearing women, to her work in both domestic and global HIV laboratory programs within DGHT.
In addition to specializing in research for HIV diagnostics and surveillance and co-leading Quality Management System (QMS) activities for her lab’s International Organization for Standardization (ISO) accreditations, Trudy has been a member of DGHT’s HIV Rapid Test Continuous Quality Improvement (RTCQI) core team which seeks to strengthen RTCQI practices in PEPFAR countries to enhance HIV rapid testing accuracy and reliability. She has specific responsibility as international subject matter expert (ISME) working with laboratorians and Ministries of Health supporting RTCQI training and program implementation in many countries of Africa and Asia. RTCQI programs she supports include tester and site certifications, integrated proficiency testing systems, and two- to three-test algorithm transitions.
Trudy looks forward to continuing to support quality management systems and innovative strategies for global HIV diagnostics and surveillance.
Kelsie Decker-Pulice

Kelsie Decker-Pulice is currently a microbiologist on the HIV Serology and Incidence Team. She focuses on international HIV serology testing, quality assurance, and various program optimization activities. She has supported several PEPFAR Population-based HIV Impact Assessments (PHIAs) in: Eswatini, Lesotho, Uganda, Malawi, Zambia, Botswana, and Tanzania by conducting lab trainings in country which focused on quality assurance in HIV rapid testing, waste management, blood collection, and sustaining the in- country laboratories well after the PHIA. Kelsie also has six years of experience in A2LA accreditation audits.
She is currently the RTCQI and serology ISME for Brazil, Ukraine, Uganda, Tanzania, Cote D’Ivoire and Lesotho and works with these countries to ensure quality testing and data usage.
Kelsie looks forward to providing technical assistance and guidance to strengthen quality management systems at the national, regional, and district levels.
Amitabh Adhikari

Amitabh Adhikari is a distinguished computer scientist currently supporting the International Laboratory Branch at the Centers for Disease Control and Prevention (CDC) in Atlanta. With a robust background in information technology, medical informatics, and public health informatics, he holds a Healthcare Information and Management Systems certification (CPHIMS) and boasts over 24 years of experience in the field. His expertise encompasses mathematical modeling and the development of information systems tailored for public and clinical laboratories.
For the past 12 years, Amitabh has played a pivotal role in providing consulting services to the Laboratory Branch and Health Informatics Team, focusing on the design, development, implementation, and support of informatics projects in PEPFAR-supported countries. His proficiency in creating IT architecture and strategic technology roadmaps has been instrumental in the effective deployment of public health solutions globally.
Amitabh's leadership experience is highlighted by his significant contributions to public health technology solutions, particularly in the context of The President's Emergency Plan for AIDS Relief (PEPFAR). He has actively engaged in health informatics initiatives, enhancing the capacity of countries to manage and respond to public health challenges.
Academically, Amitabh holds a Master's degree in Physics from the University of Delhi and a Master of Technology degree from the Indian Institute of Technology (IIT), Kanpur, India. His qualifications are further bolstered by several certifications, including:
- PMI Certified Project Management Professional (PMP)
- CompTIA Security+
- Certified Professional in Healthcare Information and Management Systems (CPHIMS)
- TOGAF 9 Certified Enterprise Architect
- Foundation Certification in Information Technology Infrastructure Library (ITIL)
- SAS Certified Advanced Programmer for SAS 9
- Certified in HL7 2.4 Controls Specialist
As he continues to advance in his career, Amitabh remains dedicated to leveraging his extensive knowledge and experience to improve public health outcomes through innovative informatics solutions.
Madelyn Baez-Santiago

Madelyn Baez-Santiago, PhD, is a microbiologist and the project lead for the Rapid Test Continuous Quality Improvement (RTCQI) initiative at the International Laboratory Branch (ILB) within CDC HQ.
She holds a doctorate in neurobiology and brings a multidisciplinary background that bridges basic science and applied public health. Her work focuses on strengthening HIV diagnostic quality through the implementation of sustainable quality assurance systems in resource-limited settings.
With extensive experience in laboratory systems, infectious disease diagnostics, and global health programs, Dr. Baez-Santiago plays a key role in advancing the accuracy and reliability of rapid testing services worldwide, contributing to critical public health research and quality improvement efforts in HIV diagnostics and testing.
Jeni Vuong

Jeni Vuong is a committed public health microbiologist with over a decade of experience in infectious disease diagnostics.
Based at the Centers for Disease Control and Prevention (CDC) in Atlanta, GA, she has provided essential expertise in bacterial meningitis and HIV. Jeni has contributed significantly to both domestic and global health efforts, including laboratory capacity strengthening, surveillance, and outbreak response for meningitis pathogens, as well as advancing innovative approaches to improve HIV diagnostic quality.
Her work has supported major global initiatives such as the Global Health Security Agenda and the President's Emergency Plan for AIDS Relief (PEPFAR).
Mervi Detorio

Mervi Detorio is currently a Microbiologist on the Serology/Incidence Team within the International Laboratory Branch (ILB) at the Division of Global HIVand TB, Center for Global Health, US Centers for Disease Control and Prevention (CDC).
Mr. Detorio earned a Bachelor of Science in Medical Technology from Manila Central University in the Philippines. He began his career in 1988 as a Research Assistant at the Research Institute for Tropical Medicine in the Philippines, where he contributed to HIV/AIDS research studies. In 1994, he joined Japan Chemical Research Pharmaceuticals, a drug discovery firm in Kobe, Japan. In 1999, he relocated to Canada and became part of Dr. Mark Wainberg’s team at the McGill University AIDS Center in Montreal, Quebec, where he conducted extensive research on HIV drug resistance. In 2003, he joined Dr. Raymond Schinazi’s group at Emory University in Atlanta, Georgia, where he continued his research on the development of HIV drug resistance before joining the International Laboratory Branch (ILB)at the CDC in 2015.
In his current role, Mr. Detorio provides technical assistance in protocol development, laboratory training, and survey implementation for HIV incidence surveillance, Population-based HIV Impact Assessment (PHIA) surveys, and HIV Rapid Recency initiatives in PEPFAR countries. He is a member of the HIV Rapid Test Continuous Quality Improvement (RTCQI) core team which seeks to enhance RTCQI practices in PEPFAR countries
Mr. Detorio has co-authored over 50 peer-reviewed publications in scientific journals, showcasing his research findings.
Robert Domaoal

Robert Domaoal is a microbiologist at the CDC’s Division of Global HIV & TB (DGHT), where he plays a key role in strengthening HIV diagnostic quality through Rapid Test Continuous Quality Improvement (RT-CQI) initiatives. As the International Subject Matter Expert (ISME) for countries including the Philippines, South Sudan, Mozambique, and Namibia, Rob provides technical leadership to enhance HIV rapid testing accuracy and reliability.
He collaborates with ministries of health, national laboratories, and implementing partners to review HIV testing algorithms, evaluate rapid test kits, and support the transition to three-test strategies. Rob has contributed to the development and review of RT-CQI training modules, external quality assessments for lay testers, and the implementation of electronic proficiency testing systems.
His work includes advising on national HIV testing policies, coordinating logistics for test kit procurement, and supporting laboratory capacity building. Rob’s efforts have directly improved testing quality and access in multiple countries, ensuring alignment with global standards and CDC guidance.
Through his RT-CQI leadership, Rob exemplifies a commitment to public health impact by advancing laboratory quality systems and supporting sustainable improvements in HIV diagnostic services worldwide.
Vedapuri Shanmugam

Dr. Vedapuri Shanmugam is a Research Biologist at the Centers for Disease Control and Prevention (CDC), working in the Serology/Incidence Laboratory within the International Laboratory Branch of the Division of Global HIV & TB (DGHT). He brings extensive expertise in HIV serology, incidence assays, early infant diagnosis (EID), viral load testing, and HIV drug resistance.
With a strong background in laboratory science and global health, Dr. Shanmugam has played a key role in supporting HIV surveillance and diagnostic capacity building in numerous countries. He has provided technical assistance and training to international partners, helping to strengthen laboratory systems and ensure the quality and reliability of HIV testing services worldwide.
His work contributes to the CDC’s mission to combat the global HIV epidemic through science, innovation, and collaboration.