Increase Uptake and Coverage of the Proficiency Testing Program
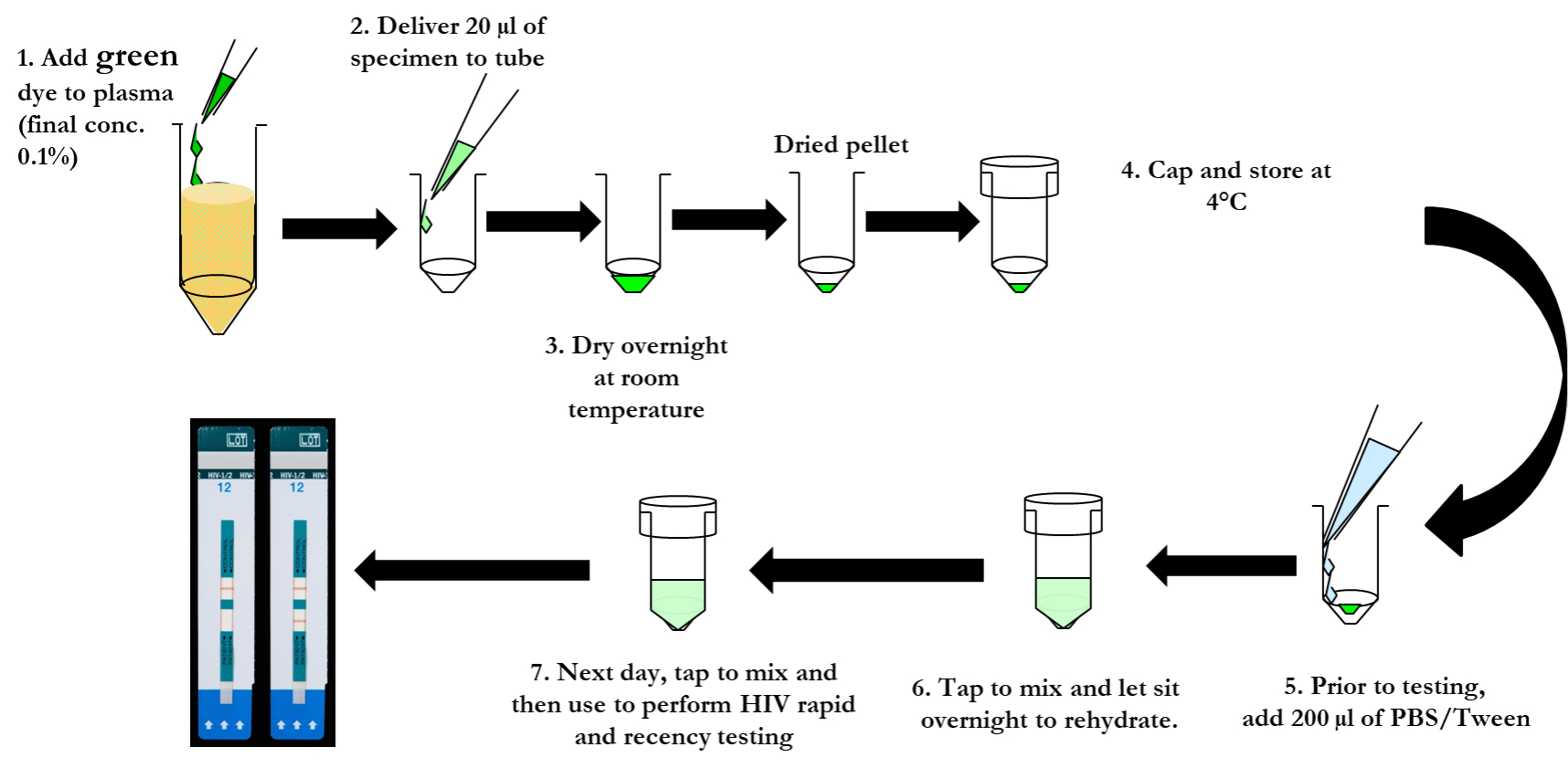
Proficiency testing (PT) is one of three integral approaches to external quality assessment and is a valuable tool in identifying poorly performing HIV testing sites. Traditional PT and quality control (QC) programs use serum and plasma specimens requiring stringent conditions, mainly cold-chain infrastructure, for storage and transportation. This has proven challenging in resource-limited settings especially with expanding the program on a national scale. In order to overcome barriers to expansion, the dried tube specimen (DTS) sample type was developed as a cost-effective approach for proficiency testing programs. The DTS-based PT program is now used widely in a number of countries and for different biomarkers. However, DTS PT uptake and coverage varies from country to country and PT data analysis and corrective action do not always occur in a timely manner.
Recognizing country limitations in completing the quality assurance cycle (QAC) for PT programs, local capacity is being strengthened by:
- Working with national reference laboratories to build their specimen repositories for PT and QC programs.
- Building human resource capacity, such as the Q-corps program, to address critical logistical challenges with PT panel distribution, return of PT results to testing sites and ensuring timely feedback and corrective actions are provided where needed.
- Providing training in a simple web-based proficiency testing data management and analysis tools to adequately monitor the sites or individuals PT performance overtime.