Ongoing Assessment of HIV Rapid Testing
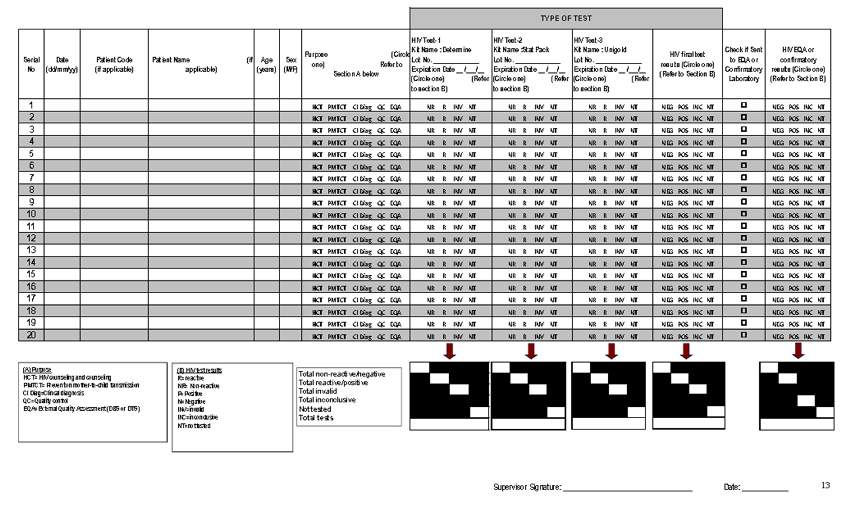
Current record keeping practices at most HIV testing sites include the use of standardized HIV testing registers that capture program related information which are not necessarily adequate for quality assurance purposes. Most registers do not include key testing quality indicators (i.e. test kit name, lot number and expiration date etc.) making it challenging to monitor the quality of testing or troubleshoot errors if needed. Most often, only a final HIV test result is entered without indicating individual test results. To address this gap, a standardized HTS test register was developed that incorporates all key testing quality information.
However, while many countries have adopted this tool, its uptake is still low and the testing quality indicators are not always reviewed for accuracy. Thus, to ensure the quality indicators captured in standardized HTS register are being reviewed and analyzed, an electronic data analysis tool has been developed which determines:
- the agreement rates between the screening and confirmatory tests used in an algorithm
- the invalid rate for each test kit used
- percentage of sites using expired test kits that may compromise the accuracy of the test results
- overall validity of the testing algorithm.
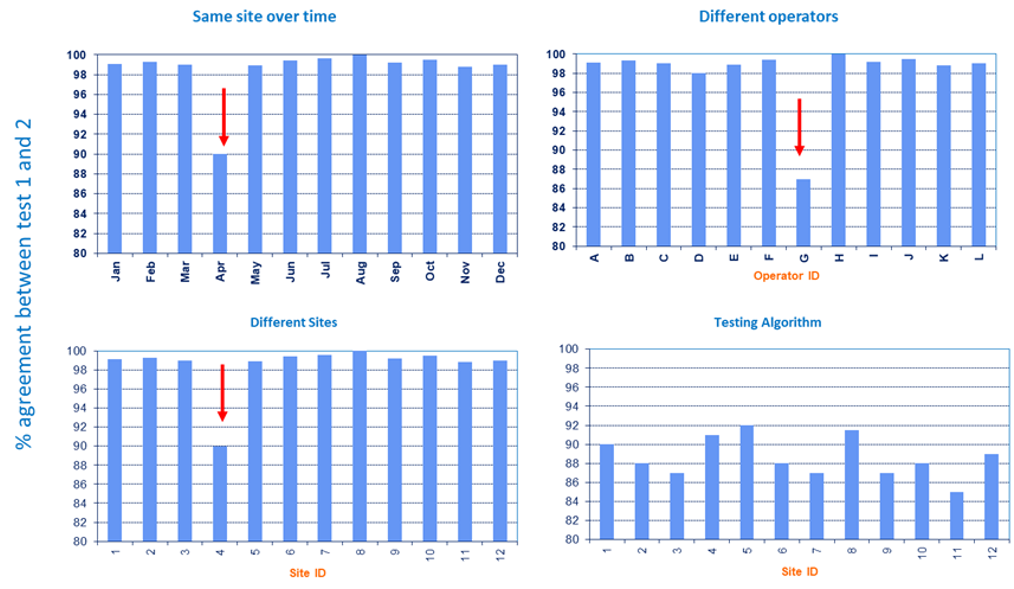
This tool is expected to be used at district level where the data can be analyzed in a timely manner and issues addressed as they arise.