Development of Pathogen-Agnostic Site Assessment Checklist for Quality Improvement of Rapid Diagnostic Testing
Background:
Site supervision is critical for ensuring quality testing, particularly in point-of-care (POC) rapid diagnostic testing. Traditionally, site supervision checklists at POC have been tailored to specific diseases and have been lacking with some diseases. This limits their adaptability across different pathogens.
To address these challenges, programs are shifting toward integrated, multi-disease testing strategies that emphasize long-term sustainability through cost-effectiveness and operational efficiency. The adaptation of a single, comprehensive tool represents a critical step forward. By moving away from siloed, disease-specific frameworks and adopting an integrated, pathogen-agnostic approach, programs can optimize resources, streamline operations, and strengthen quality improvement, especially in settings where diagnostic activities overlap.
Recognizing this challenge, the HIV SPI-RRT checklist has been adapted to be disease agnostic. This transition supports broader quality assurance frameworks that can be applied to any POC test, not just HIV rapid tests.
To date, the international laboratory (ILB) branch, working together with the Association of Public Health Laboratories (APHL) has developed a pathogen-agnostic checklist and has been piloted in two countries. In the October 2025 RTCQI CoP Session, the key presentation from APHL will highlight the experience and findings from piloting the pathogen-agnostic Stepwise Site Checklist for Rapid Testing Quality Improvement.
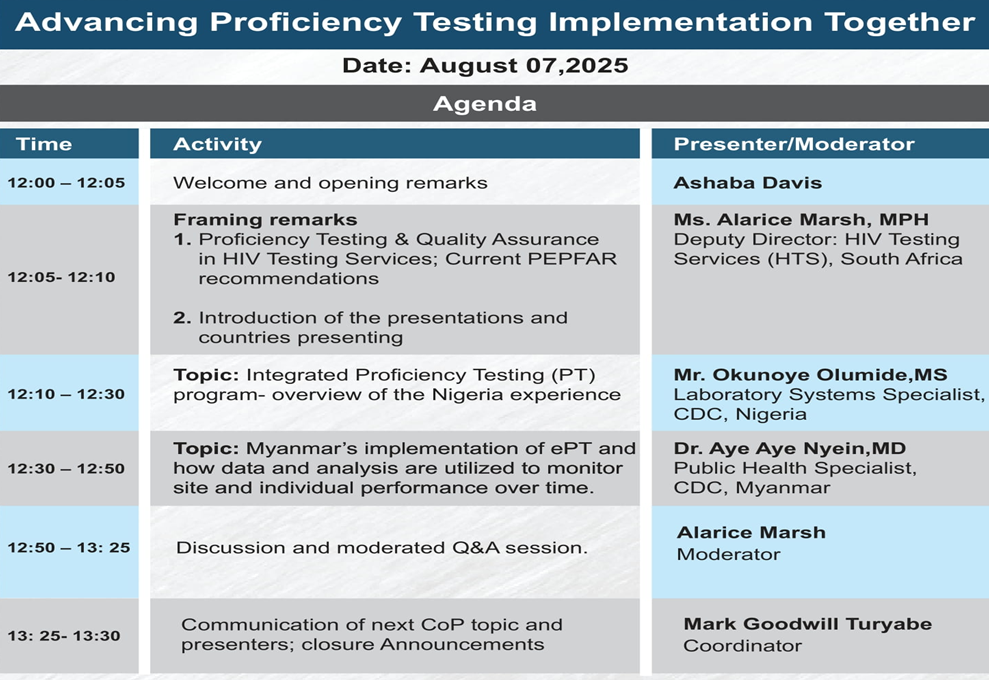
For more details, see session agenda below:
Register