Ensuring Accuracy and Sustainability in HIV Testing: Strategies and Quality Indicator Updates
Date: December 04, 2025
As the global HIV response evolves, many countries are shifting from donor-supported programs to nationally led efforts. This transition presents a valuable opportunity to enhance the sustainability and efficiency of rapid diagnostic testing, while maintaining high standards of quality. Programs are increasingly focused on optimizing resources, integrating services, and aligning quality assurance with broader health system goals.
This session will feature two presentations from the U.S. Centers for Disease Control and Prevention (CDC):
- Dr. Madelyn Baez-Santiago will highlight high-impact RTCQI strategies that promote cost efficiency and long-term sustainability.
- Ms. Jeni Vuong will present updates to quality indicators for the 3-test HIV testing algorithm. These updates support countries adopting global testing guidance for low-prevalence settings, aiming to improve diagnostic accuracy.
Following the presentations, we’ll open the line for discussion on sustainable practices, country-level innovations, and lessons learned from programs that have transitioned donor supported programs to nationally led quality assurance systems. Participants are encouraged to share experiences, challenges, and practical tips to support peer learning and inform future RTCQI efforts.
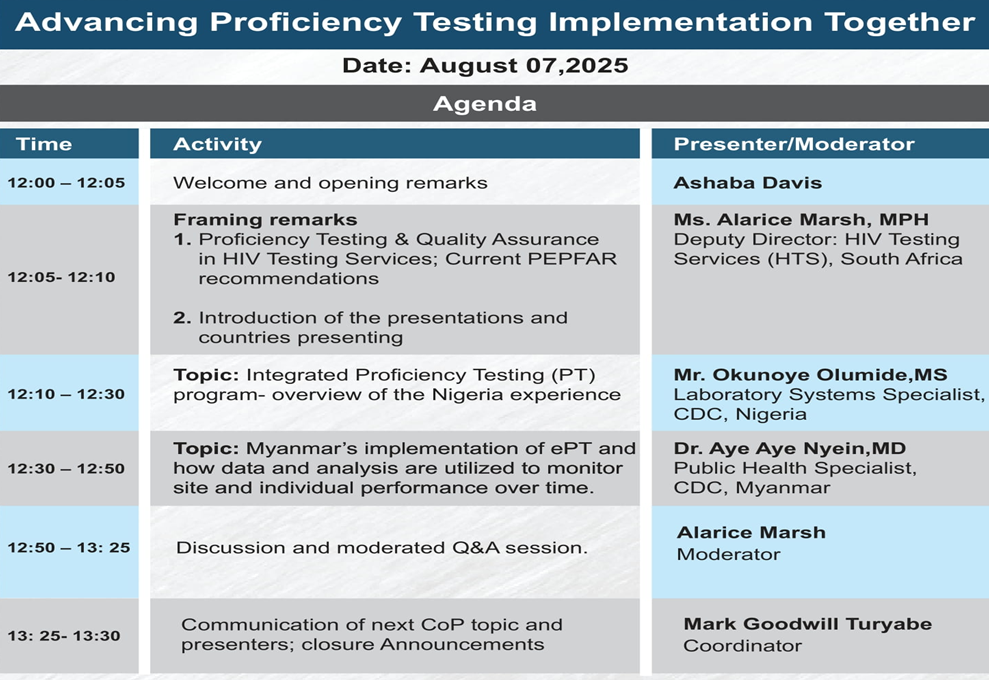
For more details, see session agenda below:
Presentations